 E. Titianova1,2, T. Chamova3, S. Karakaneva1, R. Dimova1
E. Titianova1,2, T. Chamova3, S. Karakaneva1, R. Dimova1
1Clinic of Functional Diagnostics of Nervous System, Military Medical Academy – Sofia,
2Medical Faculty, Sofia University "St. Kliment Ohridski",
3Clinic of Neurology, Alexandrovska Hospital, Medical University – Sofia
Objective: to analyze the myosonographic changes of triceps surae muscle of calves in a patient with chronic post-stroke hemiparesis.
Material and Methods: We present a 54-year-old patient with chronic right-sided hemiparesis after ischemic stroke in the left middle cerebral artery. Clinical, electromyographic and myosonographic 4-dimensional studies of both triceps surae muscles are performed. The results are compared to age and sex-matched healthy control.
Results: Clinical examination found right-sided spastic hemiparesis, hypotrophy of paretic leg and spastic-paretic gait (type Wernicke-Mann). Myosonographic study showed decreased contractility of the paretic (right) triceps surae muscle in plantar flexion and electrical stimulation. Compared to the control, the patient with stroke had bilateral changes in gastrocnemius muscle’s myoarchitectonics – the fine granular reticular structure was replaced by more coarse granular one, the perimisium hyperechoic septa of fibrous and fatty tissue were increased in size and number. The changes were significantly more pronounced on the side of paresis.
Discussion: Multimodal myosonography can objectify the structural and functional changes in triceps surae muscles in chronic hemiparesis after stroke.
Key Words: chronic hemiparesis, myosonology, stroke
It is known that although limited, the ability of the human brain to reorganize continues throughout life which is associated with brain plasticity on two functional levels: sensorimotor cortex (cortical plasticity) and neuronal network (neuronal plasticity). Changes in the central nervous system can be objectified with different functional neuroimaging and electrophysiological methods [1, 7, 15]. Recently, similar studies have established bilateral changes in motor control after stroke in which the participation of non-paretic side is proportional to the severity of brain injury [2, 3, 7, 14] and is associated with functional and structural changes in paretic muscles [6, 8, 9]. It has been shown that the myoarchitectonics of skeletal muscles may be successfully investigated by modern ultrasound techniques [12, 13, 14].
The aim of the present study was to analyze the myosonographic changes in triceps surae muscles of both legs (paretic and non-paretic) in a patient with chronic spastic hemiparesis after ischemic stroke in middle cerebral artery.
Material and Methods
A 54-year-old patient with right-sided spastic hemiparesis 1 year and 8 months after ischemic stroke in the left middle cerebral artery was studied. The cause was thrombosis of the left internal carotid artery. The severity of paresis was evaluated by manual muscle testing (MMT).
Electromyographic study was realized by Nicolet Viking Quest. Stimulation electromyography (EMG) of n. tibialis bilaterally was performed. A compound muscle action potential (CMAP) of m. gastrocnemius medialis and lateralis with bipolar surface electrodes was carried out. Standard quantitative EMG with a concentric needle electrode was done. The main characteristics of CMAP - latency time, amplitude and area of response were evaluated, as well as spontaneous activity of the muscle at rest and characteristics of motor units action potentials in mild, moderate and maximal muscle contraction.
Ultrasound characteristics of the triceps surae muscle were assessed by multi-color duplex sonography (Logic 7, GE - Germany), equipped with a transducer for 4-dimensional imaging in real time. Changes in both triceps surae muscles were measured in supine position of the patient at rest and during muscle contraction (spontaneous and induced by electric stimulation of n. tibialis) following a standard protocol [4]. The transducer was placed perpendicularly to the muscle to avoid echogenic artifacts. Qualitative and quantitative evaluation of myosonograms was performed by measuring the transverse diameter of the muscle’s two heads (lateral and medial) in longitudinal projection, the inclination of the muscle fibers to the surface of the aponeurosis and their architectonics in 4-D myosonographic imaging.
Results
Clinical examination found a right-sided hemiparesis and spastic-paretic gait (type Wernicke-Mann). There was a spastically increased muscle tone in the right limbs, hyperreflexia to polykinetic tendon reflexes and pathological reflexes of the Babinski and Rossolimo group at right. MMT showed weakness in the right limbs with flexion, abduction and adduction of the shoulder 4+/5, flexion and extension of the elbow joint 4/5, flexion and extension in the fingers and carpo-metacarpal joint 4/5. The strength of all muscle groups of his right leg was 4/5. Slight hypotrophy of the right calf was registered – circumference of 37.3 cm compared to the left one – 38.1 cm (Fig. 1).
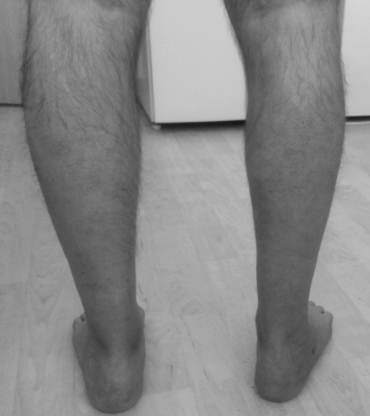
Fig. 1. Paretic hypotrophy of the right calf 20 months after a cerebral infarction in the left middle cerebral artery.
Stimulation EMG objectified normal motor conduction velocity of n. tibialis bilaterally, recording from both heads of triceps surae muscle (lateral and medial gastrocnemius muscle). Needle EMG did not show signs of denervation. Attempts for moderate and maximal plantar flexion led to registration of single action potentials with normal configuration and low amplitude from the paretic triceps surae muscle (Fig. 2). In comparison to the control motor units action potentials of non-paretic leg were with lower amplitude in maximal contraction and difficulty in sustaining contraction after the first maximum.
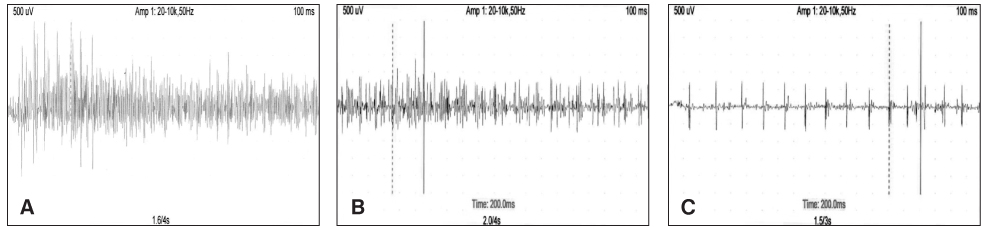
Fig. 2. EMG of m triceps surae at maximal contraction in healthy control (A) non-paretic (B) and paretic (C) leg. From paretic muscle only single action potentials are detected. Compared to the control non-paretic limb is with reduced amplitude of motor units action potentials at maximal contraction.
Compared to the non-paretic (left) calf the myosonographic study found decreased lateral diameter and reduced contractility of the right (paretic side) triceps surae muscle on plantar flexion and electrical stimulation (Fig. 3). Compared to the normal fine granular myoarchitectonics of this muscle in the control, in patients with hemiparesis bilateral asymmetrical changes in myosonograms were established, significantly more pronounced on the side of paresis - increased in number and size hyperechoic septa of fibrous and fatty tissue, more coarse granular myoarchitectonics and confluent areas in the paretic muscle (Fig. 3).
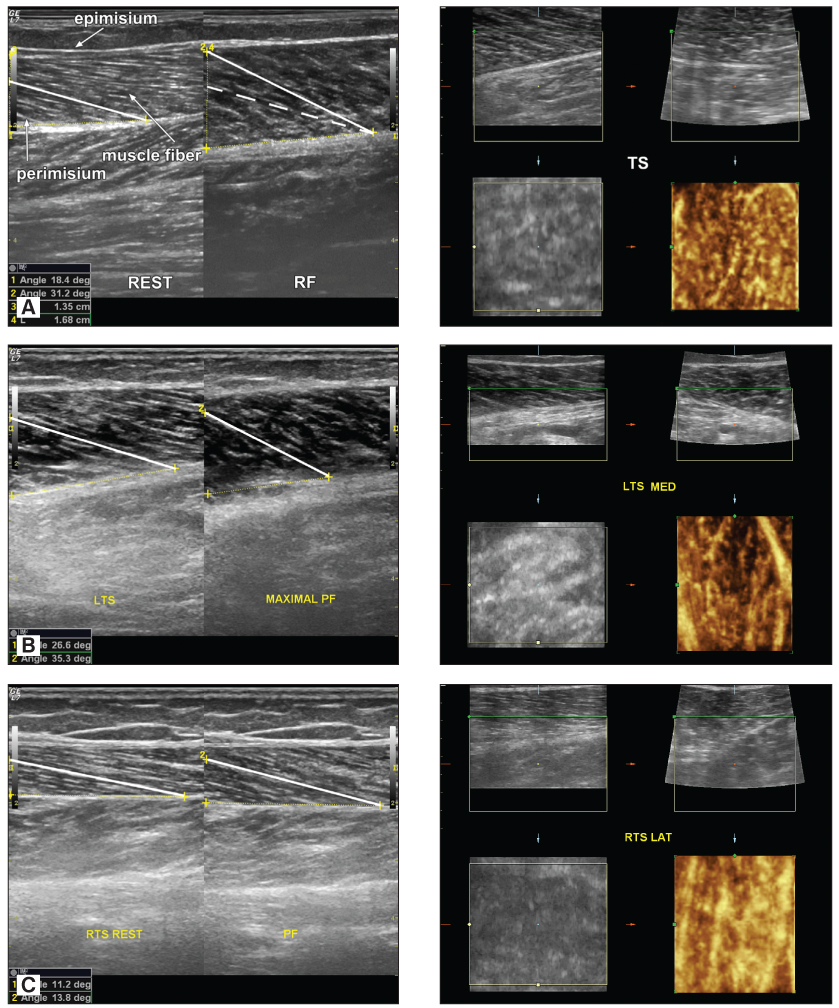
Fig. 3. Normal myosonogram of m. triceps surae in healthy subject (A) and patient with post-stroke hemiparesis (B and C). Paretic foot (C) is with reduced volume and contractility of m. triceps surae compared to non-paretic. Compared to the control in patient with stroke bilateral changes in m. triceps surae myoarchitectonics are seen – fine reticular structure is replaced by more coarse granulated one, hyperechoic septa of perimisium fibrous and fatty tissue increase in size and number, significantly expressed on the side of paresis.
Discussion
This study by four-dimensional ultrasound imaging of triceps surae muscle in post-stroke hemiparesis is the first of its kind. It establishes bilateral asymmetrical changes in triceps surae muscle myoarchitectonics (on the paretic and non-paretic side), which supports the thesis for bilateral reorganization of motor control after unilateral stroke [10, 11, 14]. The patient we presented was with decreased muscle volume of the paretic calf, asymmetric bilaterally enlarged hyperechoic septa of fibrous and fatty tissue in triceps surae perimisium and sonographic data for changed myoarchitectonics significantly expressed on the side of paresis – replacement of the normal grain grid structure of triceps surae muscle by a more coarse granular one, due to the inactivity hypotrophy, intramuscular connective tissue proliferation and fatty degeneration. Bilateral structural changes in affected muscles are established with other research methods [5-7, 9].
It is known that skeletal muscle has a large adaptive potential as myocytes can adapt to different operational loads and conditions [8]. In general, slow twitch muscle fibers (type 1) are rich in mitochondria and resistant to fatigue and fast twitch fibers (type 2) are less resistant to fatigue due to increased glycolysis processes, ensuring their energy [5]. With age, the number of muscle fibers decreases (sarcopenia) and the ratio slow/fast muscle fibers changes in favor to slow fibers [6]. Unlike changes associated with normal aging, in paretic muscle an inactivity hypotrophy, accumulation of intramuscular connective tissue, increase of collagen/muscle ratio, fat accumulation, severe deficiency of slow myosin isoforms, shortening and relative atrophy of fast muscle fibers are seen [8, 9], enhanced by physiological processes of aging. Changes in non-paretic limb are also observed – reduced muscle strength at maximum contraction [6]. By multimodal neurosonography structural and functional disturbances in triceps surae muscle after stroke become visible - they can be assessed quantitatively and displayed structurally, which is essential for early diagnosis, selection and evaluation of the therapeutic approach. However, the application of myosonology in patients who have experienced stroke is mostly experimental. The importance of the method to clinical practice is the subject of future studies.
REFERENCES
1. Попов П, Димова Р, Тодорова С, Титянова Е. Транскраниална магнитна стимулация - настояще и перспективи. Невросонология и мозъчна хемодинамика 8, 2012:22-33.
2. Титянова Е. Индикатори за двустранно променен двигателен контрол на походката при хронична хемипареза след супратенториален мозъчен инсулт. Дисертационен труд за присъждане на научна степен “доктор на науките”, С., 2007.
3. Титянова Е. Реорганизация на двигателния контрол след едностранен мозъчен инсулт. Невросонография и мозъчна хемодинамика 3, 2007:42-47.
4. Титянова Е, Гергелчева В, Михайлова В, Чамова Т, Търнев И. Миосонографни и клинико-генетични проучвания при болен с дистална миопатия. Невросонография и мозъчна хемодинамика 6, 2010:87-94
5. Bottinelli R. Functional heterogeneity of mammalian single muscle fibres: Do myosin isoforms tell the whole story? Pflugers Arch 443, 2001:6–17.
6. Gracias JM. Pathophysiology of spastic paresis. I. Paresis and soft tissue changes. Muscle Nerve 31, 2005:535-551.
7. Green JB. Brain reorganization after stroke. Fall 10, 2003:1-20.
8. Hafer-Macko С, Ryan A S, Ivey F M, Macko R F. Skeletal muscle changes after hemiparetic stroke and potential beneficial effects of exercise intervention strategies. J Rehab Res Dev 45, 2008:261-272.
9. Hortobágyi T, Dempsey L, Fraser D, Zheng D, Hamilton G, Lambert J, Dohm L. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J Physiol 524, 2000: 293–304.
10. Lotze M, Beutling W, Loibl M, Domin M, Platz T, Schminke U, Byblow WD. Contralesional motor cortex activation depends on ipsilesional corticospinal tract integrity in well-recovered subcortical stroke patients. Neurorehabil Neural Repair 26, 2012:594-603.
11. Nudo RJ, Weise BM, SiFuentes F, Milliken GW. Neuronal sub-strates for the effects of rehabilitative treating on motor recovery after ischemic infarct. Science 272, 1996:1791-1794.
12. Siebler M, Marx R, Titianova E. Myosonographie:eine aktuelle Übersicht und Ausblick. Klin Neurophysiol 43, 2012:22-26.
13. Titianova E, Chamova T, Guergueltcheva V, Tournev I. Four-dimentional ultrasound calf muscle imaging in patients with genetic types of distal myopathy. Perspectives in Medicine 1, 2012:431-434 (www.sciencedirect.com).
14. Titianova E, Peurala S, Pitkanen K, Tarkka I. Gait reveals bilateral adaptation in motor control in patients with chronic unilateral stroke. Aging Clin Exper Res 20, 2008:131-138.
15. Ward N. Assessment of cortical reorganisation for hand function after stroke. J Physiol 589, 2011 (Pt 23):5625-5632.


